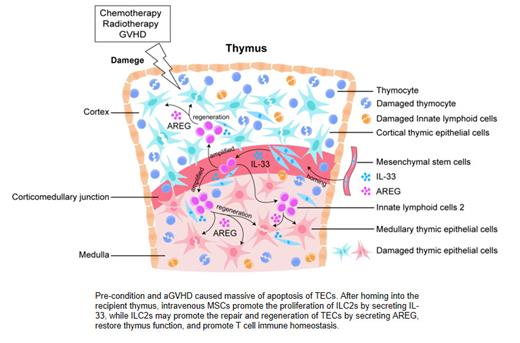
Background: Previous studies suggested that mesenchymal stromal cells (MSCs) administrated at the acute graft versus host disease (aGVHD) phase effectively decreased the incidence and severity of chronic GVHD (cGVHD) by boosting thymic regeneration. Our research has found that MSCs expressing interleukin 33 (IL33) may participate in thymus regeneration from aGVHD damage through type 2 innate lymphoid cells (ILC2s)-mediated tissue recovery. However, the mechanism for ILC2s repairing the damaged thymus caused by aGVHD is unclear. Here, we further explored the mechanisms on how ILC2s promote thymus recovery post-BMT.
Methods: We carried out studies in a aGVHD mice model of fully MHC-mismatched myeloablative bone marrow transplantation (C57BL/6 to BALB/c). IL33 knocked-down (MSC/IL33 -) and empty-load MSCs (MSC/Control) were generated by lentivirus transfection, and then administrated intravenously at day 7 post-BMT to intervene in aGVHD and assess the effect of IL33 from MSCs on ILC2s (Lin-CD45+iCD3-CD127+GATA3+) in the thymus by flow cytometery at day 14 post-BMT. Clinical scores and survival were recorded once every days. Furthermore, we isolated, purified and amplified ILC2s from C57BL/6 mouse thymus by immunomagnetic bead sorting and flow cytometry for aGVHD intervention and studied whether ILC2s can home to thymus by fluorescence tracing and the effects of ILC2s on TECs by immunofluorescence. To study the potential mechanism of ILC2s affecting TECs by using transcriptome sequencing (RNA-seq) and related molecular biology experiments. Finally, the potential target amphiregulin (AREG) was found from RNA-seq analyses and the murine recombinant AREG was used as an intervention to evaluate its effect on aGVHD mouse.
Results: In this study, we found that knocking down the expression of IL33 in MSCs partially blocked the repair effect of MSCs on thymus and decreased the expansion of thymic ILC2s in aGVHD mice compared with the MSC/Control group. Then, we found that exogenous infusion of thymic ILC2s is very effective in treating thymic tissue injury, and can home to the recipient thymus and further migrate to the thymic medulla region. Furthermore, we found that ILC2s may home to the thymus and migrate to the thymic medulla region through CCR7 and MSCs promote the high expression of AREG in ILC2s through RNA-seq. Importantly, we found that the expression level of intrathymic AREG in No-GVHD, MSCs-GVHD and ILC2s-GVHD group was significantly increased than aGVHD group on day 14 post-BMT and exogenous administration of AREG after transplantation can significantly increase the proliferation of thymic epithelial cells, especially the regeneration of thymic medulla and the generation of new thymus-derived CD4/CD8 double-positive T cells.
Conclusion: In conclusion, we demonstrate that MSCs infusion may rely on the MSCs-IL33-ILC2s-AREG-TECs axis mediated by ILC2s to remodeling the thymic microenvironment homeostasis, thus promoting the reconstruction of T cell immune homeostasis. Importantly, the results presented in this report identify a previously unrecognized role for the epidermal growth factor family member AREG in promoting the remodeling of thymus tissue. and providing new insights for the mechanism of MSCs treatment for GVHD.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal